Image-based Management of Atrial Fibrillation
Collaborating Investigators: Nassir Marrouche MD, Chris McGann MDThe CIBC Center continues to work in close collaboration with Dr. Nassir Marrouche and his team at the Comprehensive Arrhythmia Research and Management (CARMA) Center to advance the state of technology used for the monitoring and treatment of Atrial Fibrillation (AF). Atrial fibrillation (AF) is a cardiac rhythm disturbance in which the atria, the upper chambers of the heart, undergo uncontrolled and uncoordinated electrical activation so that contraction of the atria contributes almost nothing to cardiac output. While not immediately fatal (as is ventricular fibrillation) AF dramatically increases the risk of stroke, elevates mortality, and diminishes quality of life. Traditional diagnosis of AF has been limited to ECG-based determination of the time spent in this arrhythmia and there previously has been no other dependable biomarker capable of determining either the progression of the disease or suitable treatment approaches. Therapy for AF consists of either antiarrhythmic drugs that may control the arrhythmia completely or at least reduce the resulting elevated heart rate combined with anticoagulation therapy or ablation. Ablation involves destroying targeted regions of the atria with the goal of either isolating triggers of spurious electrical activity or functionally separating the atrial wall into small enough segments that the putative mechanism of the arrhythmia can no longer be sustained. The latter approach is a form of substrate stabilization, and the management of this disease has suffered from a persistent lack of means to monitor or evaluate the stability of the tissue. It is precisely in this aspect that cardiologists at the University of Utah, with support from the CIBC have made their most significant contributions.
An interdisciplinary team at the CARMA Center have made use of the segmentation, image analysis, and recently mesh generation and simulation capabilities of the CIBC to create a comprehensive program for AF management. The scope of the progress continues to expand each year and this application of CIBC technology has proven very fruitful even as it is very challenging.
The rationale and procedural goals of this DBP are as follows: (1) to develop software tools for the registration and segmentation of images from AF patients to be applied across all segments of the use of MRI in the management of AF patients; (2) to develop and validate algorithms and associated software for the quantitative analysis of preablation fibrosis and postablation edema and scarring; (3) to create compensation approaches for errors in position from heart and respiratory motion and their impact on MR images for use in real-time imaging of the heart during MRI-guided ablation; and (4) to develop and evaluate integrated visualization schemes and software for use in real-time, MRI-guided AF ablation.
 |
 |
 |
|
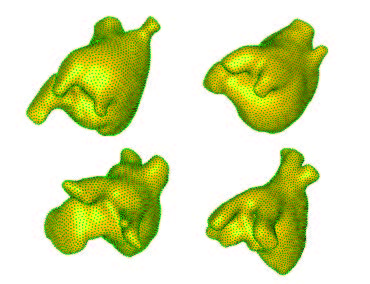
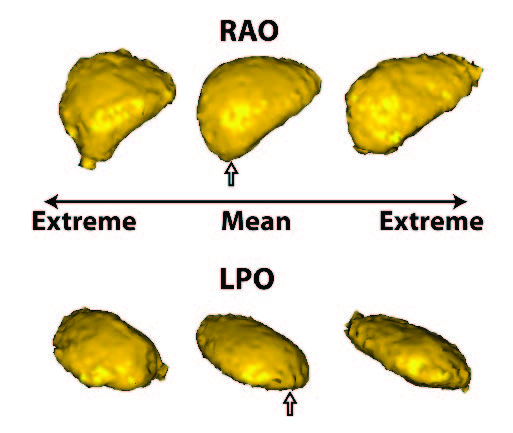
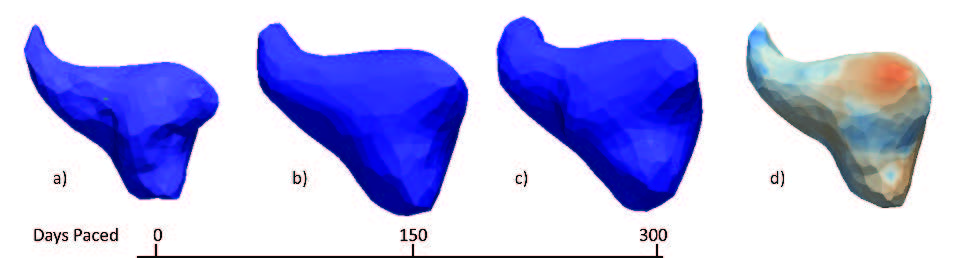
Summary of shape analysis from atrial fibrillation. Panel A shows four different left atrial shapes with the Shapeworks correspondence points marked, indicating the natural range that arises in patients. Panel B shows the results of a shape analysis of the left atrial appendage (LAA) shape in patients who all have atrial fibrillation but only a group of which proceed to experience a stroke. The images show the range of a particular mode of LAA shape variation that was statistically significantly different between patients with and without incidence of stroke. Panel C shows results from an animal model in which AF is induced over time. The progress from left to right shows how shape changes and the rightmost figure shows the locations of expansions (in red) and contractions (in blue) independent of the global size change. |
|
Progress on this DBP has continued on three main fronts:
Continued improvements in fibrosis detection and quantification through the Corview software, an application-specific derivative of Seg3D that is now the core pipeline for all MRI processing with the CARMA Center. The specific technical developments are the topic of one of the Highlights in this report. Related to this software development was the very recent Late-Breaking Clinical Trial results that Dr.~Marrouche presented at the Heart Rhythm Society Scientific Sessions conference, the largest conference in the field of cardiac electrophysiology with 10,000 participants1. In this presentation, Dr. Marrouche described the results of a 15-center, double-blinded trial of the MRI-detection of fibrosis in patients with AF. This study confirmed the results so far shown only within the CARMA Center. A manuscript on this study is now in review and should establish the scientific basis and technical feasibility of carrying out MRI-based studies of patients with AF, which CARMA has shown represent increased fibrosis in the atrial2 and are predictive of treatment success3,4.
The application of shape analysis approaches to the heart and especially the left atrium. While the volume of the left atrium has long been known to enlarge in patients with AF, our initial results suggest that more subtle features of shape are more predictive of the disease progression and even the likelihood of stroke within AF patients. These recent results were summarized in an abstract/poster at the recent Heart Rhythm Society Scientific Sessions conference5. Figure shows a summary of three applications of shape to the study of atrial fibrillation. The range of applications and the diverse use of this analysis tool make it a potentially very powerful technique that we plan to continue to exploit in both human and animal model studies.
Simulation of atrial electrical activity in both schematic and patient specific models. The scientific results from these two simulation projects are in preparation for publication of two journal articles6,7 respectively.
References:
1. N.F. Marrouche, J. Brachmann, D. Wilber, G. Hindricks, P. Jais, L. Mont, M. Duytschaever, F. Marchlinski, T. Neumann, M. Mansour, B. Herweg, E. Daoud, E. Wissner, P. Bansmann, and P. Sanders. Delayed enhancement mri determinant of successful catheter ablation for atrial fibrillation (DECAAF): A double-blinded, multi-center, prospective trial. In HRS Scientific Sessions, 2013.2. C. McGann, N. Akoum, A. Patel, E. Kholmovski, P. Revelo, K. Damal, B. Wilson, J. Cates, A. Harrison, R. Ranjan, N. Burgon, T. Greene, D. Kim, E. DiBella, D. Parker, R.S. MacLeod, and N.R. Marrouche. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on mri. Circ. Arrhythm. Electrophysiol., 2013.
3. R.S. Oakes, T.J. 3. 3. Badger, E.G. Kholmovski, N.M. Segerson, N.S. Burgon, E.N. Fish, J.E. Blauer, S.N. Rao, E.V.R. DiBella, , N. Akoum M. Daccarett, J. Winfelder, C.J. McGann, D. Parker, R.S. MacLeod, and N.F. Marrouche. Detection and quantification of left atrial structural remodeling using delayed enhancement MRI in patients with atrial fibrillation. Circ., 119(13):1758–1767, 2009.
4. N. Akoum, M. Daccarett, C. McGann, N. Segerson, G. Vergara, S. Kuppahally, T. Badger, N. Burgon, T. Haslam, E. Kholmovski, R.S. MacLeod, and N.F. Marrouche. Atrial fibrosis helps select the appropriate patient and strategy in catheter ablation of atrial fibrillation: a DE-MRI guided approach. J. Cardiovasc. Electrophysiol., 22(1):16–22, 2011.
5. G. Gardner, J. Kump, D. Chang, R.S. MacLeod, N.F. Marrouche, and J. Cates. Evaluation of left atrial appendage shape variations in atrial fibrillation patients with stroke. In Heart Rhythm Society Scientific Sessions, 2013.
6. K.S. McDowell, F. Vadakkumpadan, R. Blake, G. Planck J. Blauer, R.S. MacLeod, and N.A. Trayanova. Methodology for patient-specific modeling of atrial fibrosis as a substrate for atrial fibrillation. J. Electrocardiol., 45(6):640–645, 2012.
7. K.S. McDowell, F. Vadakkumpadan, R. Blake, J. Blauer, G. Plank, R.S. MacLeod, and N.A. Trayanova. Patient-specific modeling of atrial fibrosis reveals how fibrotic remodeling contributes to the arrhythmogenic substrate in arial fibrillation. Biophys. J. , 2013.
