SCI Publications
2021


B. Zenger, W. W. Good, J. A. Bergquist, L. C. Rupp, M. Perez, G. J. Stoddard, V. Sharma, R. S. MacLeod.
“Transient recovery of epicardial and torso ST-segment ischemic signals during cardiac stress tests: A possible physiological mechanism,” In Journal of Electrocardiology, Churchill Livingstone, 2021.
Background
Acute myocardial ischemia has several characteristic ECG findings, including clinically detectable ST-segment deviations. However, the sensitivity and specificity of diagnosis based on ST-segment changes are low. Furthermore, ST-segment deviations have been shown to be transient and spontaneously recover without any indication the ischemic event has subsided.
Objective
Assess the transient recovery of ST-segment deviations on remote recording electrodes during a partial occlusion cardiac stress test and compare them to intramyocardial ST-segment deviations.
Methods
We used a previously validated porcineBZ experimental model of acute myocardial ischemia with controllable ischemic load and simultaneous electrical measurements within the heart wall, on the epicardial surface, and on the torso surface. Simulated cardiac stress tests were induced by occluding a coronary artery while simultaneously pacing rapidly or infusing dobutamine to stimulate cardiac function. Postexperimental imaging created anatomical models for data visualization and quantification. Markers of ischemia were identified as deviations in the potentials measured at 40% of the ST-segment. Intramural cardiac conduction speed was also determined using the inverse gradient method. We assessed changes in intramyocardial ischemic volume proportion, conduction speed, clinical presence of ischemia on remote recording arrays, and regional changes to intramyocardial ischemia. We defined the peak deviation response time as the time interval after onset of ischemia at which maximum ST-segment deviation was achieved, and ST-recovery time was the interval when ST deviation returned to below thresholded of ST elevation.
Results
In both epicardial and torso recordings, the peak ST-segment deviation response time was 4.9±1.1 min and the ST-recovery time was approximately 7.9±2.5 min, both well before the termination of the ischemic stress. At peak response time, conduction speed was reduced by 50% and returned to near baseline at ST-recovery. The overall ischemic volume proportion initially increased, on average, to 37% at peak response time; however, it recovered only to 30% at the ST-recovery time. By contrast, the subepicardial region of the myocardial wall showed 40% ischemic volume at peak response time and recovered much more strongly to 25% as epicardial ST-segment deviations returned to baseline.
Conclusion
Our data show that remote ischemic signal recovery correlates with a recovery of the subepicardial myocardium, while subendocardial ischemic development persists.
2019

A. Prakosa, H.J. Arevalo, D. Deng, P.M. Boyle, P.P. Nikolov, H. Ashikaga, J.E. Blauer, E. Ghafoori, C.J. Park, R.C. Blake III, F.T. Han, R.S. MacLeod, H.R. Halperin, D.J. Callans, R. Ranjan, J. Chrispin, S. Nazarian,, N.A. Trayanova.
“Personalized Virtual-heart Technology for Guiding the Ablation of Infarct-related Ventricular Tachycardia,” In Nature Biomedical Engineering, Vol. 2, pp. 732–740. 2019.
DOI: doi.org/10.1038/s41551-018-0282-2
Ventricular tachycardia (VT), which can lead to sudden cardiac death, occurs frequently in patients with myocardial infarction. Catheter-based radio-frequency ablation of cardiac tissue has achieved only modest efficacy, owing to the inaccurate identification of ablation targets by current electrical mapping techniques, which can lead to extensive lesions and to a prolonged, poorly tolerated procedure. Here, we show that personalized virtual-heart technology based on cardiac imaging and computational modelling can identify optimal infarct-related VT ablation targets in retrospective animal (five swine) and human studies (21 patients), as well as in a prospective feasibility study (five patients). We first assessed, using retrospective studies (one of which included a proportion of clinical images with artefacts), the capability of the technology to determine the minimum-size ablation targets for eradicating all VTs. In the prospective study, VT sites predicted by the technology were targeted directly, without relying on prior electrical mapping. The approach could improve infarct-related VT ablation guidance, where accurate identification of patient-specific optimal targets could be achieved on a personalized virtual heart before the clinical procedure.
2018


B.M. Burton, K.K. Aras, W.W. Good, J.D. Tate, B. Zenger, R.S. MacLeod.
“A Framework for Image-Based Modeling of Acute Myocardial Ischemia Using Intramurally Recorded Extracellular Potentials,” In Annals of Biomedical Engineering, Springer Nature, May, 2018.
DOI: 10.1007/s10439-018-2048-0
The biophysical basis for electrocardiographic evaluation of myocardial ischemia stems from the notion that ischemic tissues develop, with relative uniformity, along the endocardial aspects of the heart. These injured regions of subendocardial tissue give rise to intramural currents that lead to ST segment deflections within electrocardiogram (ECG) recordings. The concept of subendocardial ischemic regions is often used in clinical practice, providing a simple and intuitive description of ischemic injury; however, such a model grossly oversimplifies the presentation of ischemic disease—inadvertently leading to errors in ECG-based diagnoses. Furthermore, recent experimental studies have brought into question the subendocardial ischemia paradigm suggesting instead a more distributed pattern of tissue injury. These findings come from experiments and so have both the impact and the limitations of measurements from living organisms. Computer models have often been employed to overcome the constraints of experimental approaches and have a robust history in cardiac simulation. To this end, we have developed a computational simulation framework aimed at elucidating the effects of ischemia on measurable cardiac potentials. To validate our framework, we simulated, visualized, and analyzed 226 experimentally derived acute myocardial ischemic events. Simulation outcomes agreed both qualitatively (feature comparison) and quantitatively (correlation, average error, and significance) with experimentally obtained epicardial measurements, particularly under conditions of elevated ischemic stress. Our simulation framework introduces a novel approach to incorporating subject-specific, geometric models and experimental results that are highly resolved in space and time into computational models. We propose this framework as a means to advance the understanding of the underlying mechanisms of ischemic disease while simultaneously putting in place the computational infrastructure necessary to study and improve ischemia models aimed at reducing diagnostic errors in the clinic.


B.M. Burton, K.K. Aras, W.W. Good, J.D. Tate, B. Zenger, R.S. MacLeod.
“Image-Based Modeling of Acute Myocardial Ischemia Using Experimentally Derived Ischemic Zone Source Representations,” In Journal of Electrocardiology, Vol. 51, No. 4, Elsevier BV, pp. 725--733. July, 2018.
DOI: 10.1016/j.jelectrocard.2018.05.005
Background
Computational models of myocardial ischemia often use oversimplified ischemic source representations to simulate epicardial potentials. The purpose of this study was to explore the influence of biophysically justified, subject-specific ischemic zone representations on epicardial potentials.
Methods
We developed and implemented an image-based simulation pipeline, using intramural recordings from a canine experimental model to define subject-specific ischemic regions within the heart. Static epicardial potential distributions, reflective of ST segment deviations, were simulated and validated against measured epicardial recordings.
Results
Simulated epicardial potential distributions showed strong statistical correlation and visual agreement with measured epicardial potentials. Additionally, we identified and described in what way border zone parameters influence epicardial potential distributions during the ST segment.
Conclusion
From image-based simulations of myocardial ischemia, we generated subject-specific ischemic sources that accurately replicated epicardial potential distributions. Such models are essential in understanding the underlying mechanisms of the bioelectric fields that arise during ischemia and are the basis for more sophisticated simulations of body surface ECGs.


M.J.M. Cluitmans, S. Ghimire, J. Dhamala, J. Coll-Font, J.D. Tate, S. Giffard-Roisin, J. Svehlikova, O. Doessel, M.S. Guillem, D.H. Brooks, R.S. Macleod, L. Wang.
“P1125 Noninvasive localization of premature ventricular complexes: a research-community-based approach,” In EP Europace, Vol. 20, No. Supplement, Oxford University Press, March, 2018.
DOI: 10.1093/europace/euy015.611
Background: Noninvasive localization of premature ventricular complexes (PVCs) to guide ablation therapy is one of the emerging applications of electrocardiographic imaging (ECGI). Because of its increasing clinical use, it is essential to compare the many implementations of ECGI that exist to understand the specific characteristics of each approach.
Objective: Our consortium is a community of researchers aiming to collaborate in the field of ECGI, and to objectively compare and improve methods. Here, we will compare methods to localize the origin of PVCs with ECGI.
Methods: Our consortium hosts a repository of ECGI data on its website. For the current study, participants analysed simulated electrocardiograms from premature beats, freely available on that website. These PVCs were simulated to originate from eight ventricular locations and the resulting body-surface potentials were computed. These body-surface electrocardiograms (and the torso-heart geometry) were then provided to the study participants to apply their ECGI algorithms to determine the origin of the PVCs. Participants could choose freely among four different source models, i.e., representations of the bioelectric fields reconstructed from ECGI: 1) epicardial potentials (POTepi), 2) epicardial & endocardial potentials (POTepi&endo), 3) transmembrane potentials on the endocardium and epicardium (TMPepi&endo) and 4) transmembrame potentials throughout the myocardium (TMPmyo). Participants were free to employ any software implementation of ECGI and were blinded to the ground truth data.
Results: Four research groups submitted 11 entries for this study. The figure shows the localization error between the known and reconstructed origin of each PVC for each submission, categorized per source model. Each colour represents one research group and some groups submitted results using different approaches. These results demonstrate that the variation of accuracy was larger among research groups than among the source models. Most submissions achieved an error below 2 cm, but none performed with a consistent sub-centimetre accuracy.
Conclusion: This study demonstrates a successful community-based approach to study different ECGI methods for PVC localization. The goal was not to rank research groups but to compare both source models and numerical implementations. PVC localization with these methods was not as dependent on the source representation as it was on the implementation of ECGI. Consequently, ECGI validation should not be performed on generic methods, but should be specifically performed for each lab's implementation. The novelty of this study is that it achieves this in the first open, international comparison of approaches using a common set of gold standards. Continued collaborative validation is essential to understand the effect of implementation differences, in order to reach significant improvements and arrive at clinically-relevant sub-centimetre accuracy of PVC localization.

A. Prakosa, H. J. Arevalo, D. Deng, P. M. Boyle, P. P. Nikolov, H. Ashikaga, J. J. E. Blauer, E. Ghafoori, C. J. Park, R. C. Blake, F. T. Han, R. S. MacLeod, H. R. Halperin, D. J. Callans, R. Ranjan, J. Chrispin, S. Nazarian, N. A. Trayanova.
“Personalized virtual-heart technology for guiding the ablation of infarct-related ventricular tachycardia,” In Nature Biomedical Engineering, Springer Nature America, Inc, September, 2018.
DOI: 10.1038/s41551-018-0282-2
Ventricular tachycardia (VT), which can lead to sudden cardiac death, occurs frequently in patients with myocardial infarction. Catheter-based radio-frequency ablation of cardiac tissue has achieved only modest efficacy, owing to the inaccurate identification of ablation targets by current electrical mapping techniques, which can lead to extensive lesions and to a prolonged, poorly tolerated procedure. Here, we show that personalized virtual-heart technology based on cardiac imaging and computational modelling can identify optimal infarct-related VT ablation targets in retrospective animal (five swine) and human studies (21 patients), as well as in a prospective feasibility study (five patients). We first assessed, using retrospective studies (one of which included a proportion of clinical images with artefacts), the capability of the technology to determine the minimum-size ablation targets for eradicating all VTs. In the prospective study, VT sites predicted by the technology were targeted directly, without relying on prior electrical mapping. The approach could improve infarct-related VT ablation guidance, where accurate identification of patient-specific optimal targets could be achieved on a personalized virtual heart before the clinical procedure.


A. Rodenhauser, W.W. Good, B. Zenger, J. Tate, K. Aras, B. Burton, R.S. Macleod.
“PFEIFER: Preprocessing Framework for Electrograms Intermittently Fiducialized from Experimental Recordings,” In The Journal of Open Source Software, Vol. 3, No. 21, The Open Journal, pp. 472. Jan, 2018.
DOI: 10.21105/joss.00472
Preprocessing Framework for Electrograms Intermittently Fiducialized from Experimental Recordings (PFEIFER) is a MATLAB Graphical User Interface designed to process bioelectric signals acquired from experiments.
PFEIFER was specifically designed to process electrocardiographic recordings from electrodes placed on or around the heart or on the body surface. Specific steps included in PFEIFER allow the user to remove some forms of noise, correct for signal drift, and mark specific instants or intervals in time (fiducialize) within all of the time sampled channels. PFEIFER includes many unique features that allow the user to process electrical signals in a consistent and time efficient manner, with additional options for advanced user configurations and input. PFEIFER is structured as a consolidated framework that provides many standard processing pipelines but also has flexibility to allow the user to customize many of the steps. PFEIFER allows the user to import time aligned cardiac electrical signals, semi-automatically determine fiducial markings from those signals, and perform computational tasks that prepare the signals for subsequent display and analysis.


S. Thomas, J. Silvernagel, N. Angel, E. Kholmovski, E. Ghafoori, N. Hu, J. Ashton, D.J. Dosdall, R.S. MacLeod, R. Ranjan.
“Higher contact force during radiofrequency ablation leads to a much larger increase in edema as compared to chronic lesion size,” In Journal of Cardiovascular Electrophysiology, Wiley, June, 2018.
DOI: 10.1111/jce.13636
1 Introduction
Reversible edema is a part of any radiofrequency ablation but its relationship with contact force is unknown. The goal of this study was to characterize through histology and MRI, acute and chronic ablation lesions and reversible edema with contact force.
2 Methods and results
In a canine model (n = 14), chronic ventricular lesions were created with a 3.5‐mm tip ThermoCool SmartTouch (Biosense Webster) catheter at 25 W or 40 W for 30 seconds. Repeat ablation was performed after 3 months to create a second set of lesions (acute). Each ablation procedure was followed by in vivo T2‐weighted MRI for edema and late‐gadolinium enhancement (LGE) MRI for lesion characterization. For chronic lesions, the mean scar volumes at 25 W and 40 W were 77.8 ± 34.5 mm3 (n = 24) and 139.1 ± 69.7 mm3 (n = 12), respectively. The volume of chronic lesions increased (25 W: P < 0.001, 40 W: P < 0.001) with greater contact force. For acute lesions, the mean volumes of the lesion were 286.0 ± 129.8 mm3 (n = 19) and 422.1 ± 113.1 mm3 (n = 16) for 25 W and 40 W, respectively (P < 0.001 compared to chronic scar). On T2‐weighted MRI, the acute edema volume was on average 5.6–8.7 times higher than the acute lesion volume and increased with contact force (25 W: P = 0.001, 40 W: P = 0.011).
3 Conclusion
With increasing contact force, there is a marginal increase in lesion size but accompanied with a significantly larger edema. The reversible edema that is much larger than the chronic lesion volume may explain some of the chronic procedure failures.
2017


E. Ghafoori, E.G. Kholmovski, S. Thomas, J. Silvernagel, N. Angel, N. Hu, D.J. Dosdall, R.s. MacLeod, R. Ranjan.
“Characterization of Gadolinium Contrast Enhancement of Radiofrequency Ablation Lesions in Predicting Edema and Chronic Lesion Size,” In Circulation: Arrhythmia and Electrophysiology, Vol. 10, No. 11, Ovid Technologies (Wolters Kluwer Health), pp. e005599. Oct, 2017.
DOI: 10.1161/circep.117.005599
Background Magnetic resonance imaging (MRI) has been used to acutely visualize radiofrequency ablation lesions, but its accuracy in predicting chronic lesion size is unknown. The main goal of this study was to characterize different areas of enhancement in late gadolinium enhancement MRI done immediately after ablation to predict acute edema and chronic lesion size.
Methods and Results In a canine model (n=10), ventricular radiofrequency lesions were created using ThermoCool SmartTouch (Biosense Webster) catheter. All animals underwent MRI (late gadolinium enhancement and T2-weighted edema imaging) immediately after ablation and after 1, 2, 4, and 8 weeks. Edema, microvascular obstruction, and enhanced volumes were identified in MRI and normalized to chronic histological volume. Immediately after contrast administration, the microvascular obstruction region was 3.2±1.1 times larger than the chronic lesion volume in acute MRI. Even 60 minutes after contrast administration, edema was 8.7±3.31 times and the enhanced area 6.14±2.74 times the chronic lesion volume. Exponential fit to the microvascular obstruction volume was found to be the best predictor of chronic lesion volume at 26.14 minutes (95% prediction interval, 24.35–28.11 minutes) after contrast injection. The edema volume in late gadolinium enhancement correlated well with edema volume in T2-weighted MRI with an R2 of 0.99.
Conclusion Microvascular obstruction region on acute late gadolinium enhancement images acquired 26.1 minutes after contrast administration can accurately predict the chronic lesion volume. We also show that T1-weighted MRI images acquired immediately after contrast injection accurately shows edema resulting from radiofrequency ablation.


S. Ghimire, J. Dhamala, J. Coll-Font, J. D. Tate, M. S. Guillem, D. H. Brooks, R. S. MacLeod, L. Wang.
“Overcoming Barriers to Quantification and Comparison of Electrocardiographic Imaging Methods: A Community-Based Approach,” In Computing in Cardiology, Vol. 44, 2017.
There has been a recent upsurge in the development of electrocardiographic imaging (ECGI) methods, along with a significant increase in clinical application. To better assess the state-of-the-art, enable reliable progress, and facilitate clinical adoption, it is important to be able to compare results in a comprehensive manner, scientifically and clinically. However, studies vary in modeling choices, computational methods, validation mechanisms and metrics, and clinical applications, making unified evaluation and comparison of ECGI a critical challenge.
This paper describes initial results of a project to address this challenge via a community-based approach organized by the Consortium for Electrocardiographic Imaging (CEI). We detail different aspects of this collective effort including a data sharing repository, a platform for comparison of different algorithms and modeling approaches on the same datasets, several active workgroups and progress made along these directions. We also summarize the results from groups participating in this collaboration and contributing solutions by applying their methods to the same dataset for comparison.


W. W. Good, B. Erem, J. Coll-Font, D. H. Brooks, R. S. MacLeod.
“Detecting Ischemic Stress to the Myocardium Using Laplacian Eigenmaps and Changes to Conduction Velocity,” In Computing in Cardiology, Vol. 44, IEEE, 2017.
The underlying pathophysiology of ischemia and its electrocardiographic consequences are poorly understood, resulting in unreliable diagnosis of this disease. This limited knowledge of underlying mechanisms suggests a data driven approach, which seeks to identify patterns in the ECG that can be linked statistically to underlying behavior and conditions of ischemic stress. The gold standard ECG metrics for evaluating ischemia monitor vertical deflections within the ST segment. However, ischemia influences all portions of the electrogram. Another metric that targets the QRS complex during ischemia is Conduction Velocity (CV). An even more inclusive, data driven approach is known as "Laplacian Eigenmaps" (LE), which can identify trajectories, or "manifolds", that respond to different spatiotemporal consequences of ischemic stress, and these changes to the trajectories on the manifold may serve as a clinically relevant biomarker. On this study, we compared the LE- and CV-based markers against two gold standards for detecting ischemic stress, both derived from the ST segment. We evaluated the response time and fidelity of each biomarker using a Time to Threshold (TTT) and Contrast Ratio (CR) measure, over 51 episodes recorded as cardiac electrograms from a canine model of controlled ischemia. The results show that metrics designed to monitor regions beyond the ST segment can perform at least as well, if not better, than traditional ST segment based metrics.
2016


K. Aras B. Burton, D. Swenson, R.S. MacLeod.
“Spatial organization of acute myocardial ischemia,” In Journal of Electrocardiology, Vol. 49, No. 3, Elsevier, pp. 323–336. May, 2016.
Introduction
Myocardial ischemia is a pathological condition initiated by supply and demand imbalance of the blood to the heart. Previous studies suggest that ischemia originates in the subendocardium, i.e., that nontransmural ischemia is limited to the subendocardium. By contrast, we hypothesized that acute myocardial ischemia is not limited to the subendocardium and sought to document its spatial distribution in an animal preparation. The goal of these experiments was to investigate the spatial organization of ischemia and its relationship to the resulting shifts in ST segment potentials during short episodes of acute ischemia.
Methods
We conducted acute ischemia studies in open-chest canines (N = 19) and swines (N = 10), which entailed creating carefully controlled ischemia using demand, supply or complete occlusion ischemia protocols and recording intramyocardial and epicardial potentials. Elevation of the potentials at 40% of the ST segment between the J-point and the peak of the T-wave (ST40%) provided the metric for local ischemia. The threshold for ischemic ST segment elevations was defined as two standard deviations away from the baseline values.
Results
The relative frequency of occurrence of acute ischemia was higher in the subendocardium (78% for canines and 94% for swines) and the mid-wall (87% for canines and 97% for swines) in comparison with the subepicardium (30% for canines and 22% for swines). In addition, acute ischemia was seen arising throughout the myocardium (distributed pattern) in 87% of the canine and 94% of the swine episodes. Alternately, acute ischemia was seen originating only in the subendocardium (subendocardial pattern) in 13% of the canine episodes and 6% of the swine episodes (p < 0.05).
Conclusions
Our findings suggest that the spatial distribution of acute ischemia is a complex phenomenon arising throughout the myocardial wall and is not limited to the subendocardium.


B. Erem, R.M. Orellana, D.E. Hyde, J.M. Peters, F.H. Duffy, P. Stovicek, S.K. Warfield, R.S. MacLeod, G. Tadmor, D.H. Brooks.
“Extensions to a manifold learning framework for time-series analysis on dynamic manifolds in bioelectric signals,” In Physical Review E, Vol. 93, No. 4, American Physical Society, apr, 2016.
DOI: 10.1103/physreve.93.042218
This paper addresses the challenge of extracting meaningful information from measured bioelectric signals generated by complex, large scale physiological systems such as the brain or the heart. We focus on a combination of the well-known Laplacian eigenmaps machine learning approach with dynamical systems ideas to analyze emergent dynamic behaviors. The method reconstructs the abstract dynamical system phase-space geometry of the embedded measurements and tracks changes in physiological conditions or activities through changes in that geometry. It is geared to extract information from the joint behavior of time traces obtained from large sensor arrays, such as those used in multiple-electrode ECG and EEG, and explore the geometrical structure of the low dimensional embedding of moving time windows of those joint snapshots. Our main contribution is a method for mapping vectors from the phase space to the data domain. We present cases to evaluate the methods, including a synthetic example using the chaotic Lorenz system, several sets of cardiac measurements from both canine and human hearts, and measurements from a human brain.


S. Guler, M. Dannhauer, B. Erem, R.S. Macleod, D. Tucker, S. Turovets, P. Luu, D. Erdogmus, D. Brooks.
“Optimization of focality and direction in dense electrode array transcranial direct currentstimulation (tDCS),” In Journal of Neural Engineering, Vol. 13, No. 3, IOP Publishing, pp. 036020. May, 2016.
DOI: 10.1088/1741-2560/13/3/036020
OBJECTIVE:
Transcranial direct current stimulation (tDCS) aims to alter brain function non-invasively via electrodes placed on the scalp. Conventional tDCS uses two relatively large patch electrodes to deliver electrical current to the brain region of interest (ROI). Recent studies have shown that using dense arrays containing up to 512 smaller electrodes may increase the precision of targeting ROIs. However, this creates a need for methods to determine effective and safe stimulus patterns as the number of degrees of freedom is much higher with such arrays. Several approaches to this problem have appeared in the literature. In this paper, we describe a new method for calculating optimal electrode stimulus patterns for targeted and directional modulation in dense array tDCS which differs in some important aspects with methods reported to date.
APPROACH:
We optimize stimulus pattern of dense arrays with fixed electrode placement to maximize the current density in a particular direction in the ROI. We impose a flexible set of safety constraints on the current power in the brain, individual electrode currents, and total injected current, to protect subject safety. The proposed optimization problem is convex and thus efficiently solved using existing optimization software to find unique and globally optimal electrode stimulus patterns.
MAIN RESULTS:
Solutions for four anatomical ROIs based on a realistic head model are shown as exemplary results. To illustrate the differences between our approach and previously introduced methods, we compare our method with two of the other leading methods in the literature. We also report on extensive simulations that show the effect of the values chosen for each proposed safety constraint bound on the optimized stimulus patterns.
SIGNIFICANCE:
The proposed optimization approach employs volume based ROIs, easily adapts to different sets of safety constraints, and takes negligible time to compute. An in-depth comparison study gives insight into the relationship between different objective criteria and optimized stimulus patterns. In addition, the analysis of the interaction between optimized stimulus patterns and safety constraint bounds suggests that more precise current localization in the ROI, with improved safety criterion, may be achieved by careful selection of the constraint bounds.


I.A. Polejaeva, R. Ranjan, C.J. Davies, M. Regouski, J. Hall, A.L. Olsen, Q. Meng, H.M. Rutigliano, D.J. Dosdall, N.A. Angel, F.B. Sachse, T. Seidel, A.J. Thomas, R. Stott, K.E. Panter, P.M. Lee, A.J. Van Wettere, J.R. Stevens, Z. Wang, R.S. Macleod, N.F. Marrouche, K.L. White.
“Increased Susceptibility to Atrial Fibrillation Secondary to Atrial Fibrosis in Transgenic Goats Expressing Transforming Growth Factor-β1,” In Journal of Cardiovascular Electrophysiology, Vol. 27, No. 10, Wiley-Blackwell, pp. 1220--1229. Aug, 2016.
DOI: 10.1111/jce.13049
Introduction
Large animal models of progressive atrial fibrosis would provide an attractive platform to study relationship between structural and electrical remodeling in atrial fibrillation (AF). Here we established a new transgenic goat model of AF with cardiac specific overexpression of TGF-β1 and investigated the changes in the cardiac structure and function leading to AF.
Methods and Results
Transgenic goats with cardiac specific overexpression of constitutively active TGF-β1 were generated by somatic cell nuclear transfer. We examined myocardial tissue, ECGs, echocardiographic data, and AF susceptibility in transgenic and wild-type control goats. Transgenic goats exhibited significant increase in fibrosis and myocyte diameters in the atria compared to controls, but not in the ventricles. P-wave duration was significantly greater in transgenic animals starting at 12 months of age, but no significant chamber enlargement was detected, suggesting conduction slowing in the atria. Furthermore, this transgenic goat model exhibited a significant increase in AF vulnerability. Six of 8 transgenic goats (75%) were susceptible to AF induction and exhibited sustained AF (>2 minutes), whereas none of 6 controls displayed sustained AF (P < 0.01). Length of induced AF episodes was also significantly greater in the transgenic group compared to controls (687 ± 212.02 seconds vs. 2.50 ± 0.88 seconds, P < 0.0001), but no persistent or permanent AF was observed.
Conclusion
A novel transgenic goat model with a substrate for AF was generated. In this model, cardiac overexpression of TGF-β1 led to an increase in fibrosis and myocyte size in the atria, and to progressive P-wave prolongation. We suggest that these factors underlie increased AF susceptibility.
2015


K.K. Aras, W. Good, J. Tate, B.M. Burton, D.H. Brooks, J. Coll-Font, O. Doessel, W. Schulze, D. Patyogaylo, L. Wang, P. Van Dam,, R.S. MacLeod.
“Experimental Data and Geometric Analysis Repository: EDGAR,” In Journal of Electrocardiology, 2015.
Introduction
The "Experimental Data and Geometric Analysis Repository", or EDGAR is an Internet-based archive of curated data that are freely distributed to the international research community for the application and validation of electrocardiographic imaging (ECGI) techniques. The EDGAR project is a collaborative effort by the Consortium for ECG Imaging (CEI, ecg-imaging.org), and focused on two specific aims. One aim is to host an online repository that provides access to a wide spectrum of data, and the second aim is to provide a standard information format for the exchange of these diverse datasets.
Methods
The EDGAR system is composed of two interrelated components: 1) a metadata model, which includes a set of descriptive parameters and information, time signals from both the cardiac source and body-surface, and extensive geometric information, including images, geometric models, and measure locations used during the data acquisition/generation; and 2) a web interface. This web interface provides efficient, search, browsing, and retrieval of data from the repository.
Results
An aggregation of experimental, clinical and simulation data from various centers is being made available through the EDGAR project including experimental data from animal studies provided by the University of Utah (USA), clinical data from multiple human subjects provided by the Charles University Hospital (Czech Republic), and computer simulation data provided by the Karlsruhe Institute of Technology (Germany).
Conclusions
It is our hope that EDGAR will serve as a communal forum for sharing and distribution of cardiac electrophysiology data and geometric models for use in ECGI research.


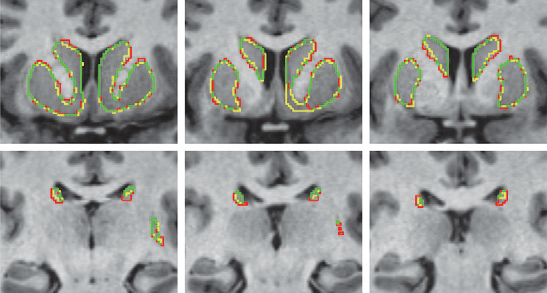
Y. Gao, L. Zhu, J. Cates, R. S. MacLeod, S. Bouix,, A. Tannenbaum.
“A Kalman Filtering Perspective for Multiatlas Segmentation,” In SIAM J. Imaging Sciences, Vol. 8, No. 2, pp. 1007-1029. 2015.
DOI: 10.1137/130933423
In multiatlas segmentation, one typically registers several atlases to the novel image, and their respective segmented label images are transformed and fused to form the final segmentation. In this work, we provide a new dynamical system perspective for multiatlas segmentation, inspired by the following fact: The transformation that aligns the current atlas to the novel image can be not only computed by direct registration but also inferred from the transformation that aligns the previous atlas to the image together with the transformation between the two atlases. This process is similar to the global positioning system on a vehicle, which gets position by inquiring from the satellite and by employing the previous location and velocity—neither answer in isolation being perfect. To solve this problem, a dynamical system scheme is crucial to combine the two pieces of information; for example, a Kalman filtering scheme is used. Accordingly, in this work, a Kalman multiatlas segmentation is proposed to stabilize the global/affine registration step. The contributions of this work are twofold. First, it provides a new dynamical systematic perspective for standard independent multiatlas registrations, and it is solved by Kalman filtering. Second, with very little extra computation, it can be combined with most existing multiatlas segmentation schemes for better registration/segmentation accuracy.


K. Gillette, J.D. Tate, B. Kindall, P. Van Dam, E. Kholmovski, R.S. MacLeod.
“Generation of Combined-Modality Tetrahedral Meshes,” In Computing in Cardiology, 2015.
Registering and combining anatomical components from different image modalities, like MRI and CT that have different tissue contrast, could result in patient-specific models that more closely represent underlying anatomical structures.
In this study, we combined a pair of CT and MRI scans of a pig thorax to make a tetrahedral mesh and compared different registration techniques including rigid, affine, thin plate spline morphing (TPSM), and iterative closest point (ICP), to superimpose the segmented bones from the CT scan on the soft tissues segmented from the MRI. The TPSM and affine-registered bones remained close to, but not overlapping, important soft tissue.
Simulation models, including an ECG forward model and a defibrillation model, were computed on generated multi-modality meshes after TPSM and affine registration and compared to those based on the original torso mesh.


K.S. McDowell, S. Zahid, F. Vadakkumpadan, J.J. Blauer, R.S. MacLeod, N.A. Trayanova.
“Virtual Electrophysiological Study of Atrial Fibrillation in Fibrotic Remodeling,” In PLoS ONE, Vol. 10, No. 2, pp. e0117110. February, 2015.
DOI: 10.1371/journal.pone.0117110
Research has indicated that atrial fibrillation (AF) ablation failure is related to the presence of atrial fibrosis. However it remains unclear whether this information can be successfully used in predicting the optimal ablation targets for AF termination. We aimed to provide a proof-of-concept that patient-specific virtual electrophysiological study that combines i) atrial structure and fibrosis distribution from clinical MRI and ii) modeling of atrial electrophysiology, could be used to predict: (1) how fibrosis distribution determines the locations from which paced beats degrade into AF; (2) the dynamic behavior of persistent AF rotors; and (3) the optimal ablation targets in each patient. Four MRI-based patient-specific models of fibrotic left atria were generated, ranging in fibrosis amount. Virtual electrophysiological studies were performed in these models, and where AF was inducible, the dynamics of AF were used to determine the ablation locations that render AF non-inducible. In 2 of the 4 models patient-specific models AF was induced; in these models the distance between a given pacing location and the closest fibrotic region determined whether AF was inducible from that particular location, with only the mid-range distances resulting in arrhythmia. Phase singularities of persistent rotors were found to move within restricted regions of tissue, which were independent of the pacing location from which AF was induced. Electrophysiological sensitivity analysis demonstrated that these regions changed little with variations in electrophysiological parameters. Patient-specific distribution of fibrosis was thus found to be a critical component of AF initiation and maintenance. When the restricted regions encompassing the meander of the persistent phase singularities were modeled as ablation lesions, AF could no longer be induced. The study demonstrates that a patient-specific modeling approach to identify non-invasively AF ablation targets prior to the clinical procedure is feasible.


K. S. McDowell, S. Zahid, F. Vadakkumpadan, J. Blauer, R. S. MacLeod, N. A. Trayanova.
“Virtual Electrophysiological Study of Atrial Fibrillation in Fibrotic Remodeling,” In PLoS ONE, Vol. 10, No. 2, Public Library of Science, pp. 1-16. May, 2015.
DOI: doi.org/10.1371/journal.pone.0117110
Research has indicated that atrial fibrillation (AF) ablation failure is related to the presence of atrial fibrosis. However it remains unclear whether this information can be successfully used in predicting the optimal ablation targets for AF termination. We aimed to provide a proof-of-concept that patient-specific virtual electrophysiological study that combines i) atrial structure and fibrosis distribution from clinical MRI and ii) modeling of atrial electrophysiology, could be used to predict: (1) how fibrosis distribution determines the locations from which paced beats degrade into AF; (2) the dynamic behavior of persistent AF rotors; and (3) the optimal ablation targets in each patient. Four MRI-based patient-specific models of fibrotic left atria were generated, ranging in fibrosis amount. Virtual electrophysiological studies were performed in these models, and where AF was inducible, the dynamics of AF were used to determine the ablation locations that render AF non-inducible. In 2 of the 4 models patient-specific models AF was induced; in these models the distance between a given pacing location and the closest fibrotic region determined whether AF was inducible from that particular location, with only the mid-range distances resulting in arrhythmia. Phase singularities of persistent rotors were found to move within restricted regions of tissue, which were independent of the pacing location from which AF was induced. Electrophysiological sensitivity analysis demonstrated that these regions changed little with variations in electrophysiological parameters. Patient-specific distribution of fibrosis was thus found to be a critical component of AF initiation and maintenance. When the restricted regions encompassing the meander of the persistent phase singularities were modeled as ablation lesions, AF could no longer be induced. The study demonstrates that a patient-specific modeling approach to identify non-invasively AF ablation targets prior to the clinical procedure is feasible.