Lab 2 : Frog Heart Experiment March 2nd, 2009
Dafang Wang
1. Introduction
The heart is adapted to adjust its activities and output in order to meet the physiological requirements of organisms in response to external environments. There are basically two ways for the heart to achieve its adaption: the heart beat rate and the contraction strength. Controlling this physiological process is a variety of mechanical and chemical factors, whose respective influence is to be explored by this experiment.
The purpose of the experiment is to investigate the effects of mechanical and chemical stimuli on the function of cardiac muscles. First, various levels of mechanical preload were applied on the heart in order to assess its responsive contraction strength. Second, different chemical stimulants with anticipated effects were placed on the heart surface while the resulting ECG signals were monitored.
2. Method
2.1. Instrument Calibration
This experiment used a force transducer to assess the contraction force of the heart. Because the transducer gave electrical signals, calibration was needed both to remove the instrumental deflection and to transform electrical signals to forces to be considered in the test.
The calibration was carried out by hanging on the transducer several clips of known weights and recording the electric voltages returned by the transducer. The weights were supposed to be in proportion to the voltages. In this way, we were able to obtain the force value when measuring the contraction strength later.
Figure 1 presents the calibration and the relationship between the voltages and the testing tension force. The linear regression fitted the data with an accuracy rate of 0.9986. We used the formulation in Figure 1 to calculate the forces measured throughout this laboratory.
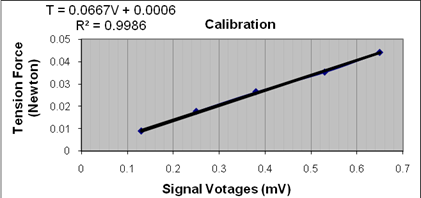
Figure 1. Calibration of the transducer reading (voltage) and the force measured. The relationship between the two quantities is given on the top left corner, where T denotes the force and V represents the voltage.
2.2. Preparation of the specimen
As shown in Figure 2, a pitched frog was secured on the pan, with its thorax being cut open so as to expose the heart. The lower part of the ventricle was tied to a suture thread, which was then connected with the transducer blade.


Figure 2: Illustration of the frog preparation. A suture thread connected the heart ventricle with the transducer (right).
2.3. Response to Stretch Test
The tension on the thread connecting the heart and the transducer was initially set to be just enough to obtain the contraction signal. The tension was incrementally increased by moving the pan further from the transducer, while the contraction strength was recorded at each step. The maximum contraction strength was evaluated by the difference between the peak tension and the pre-tension before and after the heart contraction. The maximum contraction strength was to be plotted versus the pre-tension in order to explore the Starling mechanism.
2.4. Response to Chemical Stimulants Test
Various types of chemicals were applied on the heart surface, and the resulting tension and heart rate were collected for evaluation of the heartí»s response. Ringerí»s solution was used on the heart after each chemical test was finished so as to eliminating the remaining influence of preceding chemicals.
2.5. Data Processing
Matlab scripts were developed to detect the features of interest in the recording signals, such as the peak tension in contraction, the baseline pre-tension, and the instantaneous heart rates.
3. Results
3.1 Response to stretch
The connection between the frog and the transducer was initially set to just allow the detection of the heart contraction. The recorded signals served as the baseline of this experiment. The distance was then incremented in 9 steps to increase the pre-load tension on ventricles. The recorded tension forces are shown in Figure 3(left). The temporal variance of each signal marks the contraction/relaxation of cardiac cycles. In each signal, the minima represent the pre-tension, whereas the maxima mean the tensions measured in peak contractions. As the pre-tension increased with the distance, the overall tension consistently rose. The amplitude of the tension signal increased, too.
This amplitude, which is the difference between the force at the peak contraction and the pre-tension, implies the peak contraction strength of the heart. Figure 3(right) demonstrates the positive correlation between the peak contraction strength and the pre-tension.
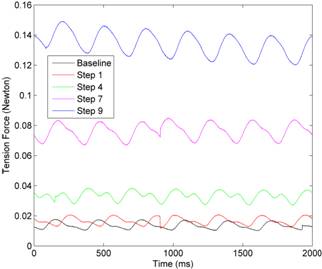
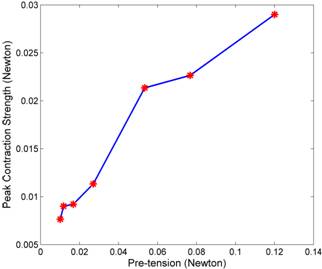
Figure 3. Illustration of the impact of pre-loading on contraction strength. (Left): tension force signals measured in several step. The temporal variance indicates the contraction/release cycle of the heartbeat. (Right): the peak contraction strength collected under different levels of pre-tension.
3.2. Response to Chemical Stimulants
![]() 3.2.1. 2 C Ringerí»s solution
3.2.1. 2 C Ringerí»s solution
As shown in Figure 4, the cold Ringerí»s solution reduced the heart contraction force as well as the amplitude of ECG voltage signals. The instantaneous heartbeat rate dropped from 30BPM to about 26 BPM.

Figure 4. (Left): heart contractions before and after applying cold Ringerí»s solution. (Right): Corresponding ECG voltages.
3.2.2 Caffeine
Following the application of 30mM of caffeine, the heartbeat rate dropped from the baseline of 30BPM to 27BPM. As shown in Figure 5, the contraction force fell by half of the original strength. The amplitude of ECG signals also decreased in terms of the amplitude of its QRS segment.
However, the caffeine is supposed to increase the strength and frequency of heart beats. This experiment failed to collect correct data. One possible reason is we had not waited long enough for the caffeine to take effect.
3.2.3. Cadmium Chloride
As shown in Figure 6, applying cadmium chloride moderately attenuated heart contraction strengths. Significant changes were observed in the patterns of P-wave and QRS segments in ECG signals. Compared to the normal condition, the P-wave in this test was notably elevated to positive values, while the QRS segment shortened its duration and an additional S-spike appeared. The heart rate rose slightly from 30 BPM to 32-33 BPM.
3.2.4. Epinphrine
As shown in Figure 7, heat contractions were moderately strengthened following the introduction of epinephrine, while no noticeable change was seen in the heartbeat rate, which was about 30 BPM at the baseline. The magnitude of the ECG signal was increased.

Figure 5. Heart contractions and ECG signals before and after applying caffeine on the heart surface.

Figure 6. Heart contractions (left) and ECG signals (right) before and after applying Cadmium Chloride.

Figure 7. Heart contractions(left) and the ECG voltages(right) before and after applying epinephrine.
3.2.5. 5µM Ach
After applying ACh solutions, the strength of heart contractions dropped from 0.035N to 0.02N (Figure 8(left)). The heart rate fell from 30 BPM at the baseline to 18 BPM, a distinctive decrease in contrast to other chemicals. The right panel of Figure 8 shows the ECG signal was also amplified in magnitude.
We noted that the baseline signal and contractions became much weaker from then on compared to what they had been at the beginning of the experiment, as can be observed by comparing Figure 5 and Figure 8. The heart bad been exposed to tests for two hours by that time, and it could not maintain normal activities for lack of nutrition and oxygen. In fact, the purpose of adding normal Ringerí»s solution onto the heart after each test was to prolong its active duration.
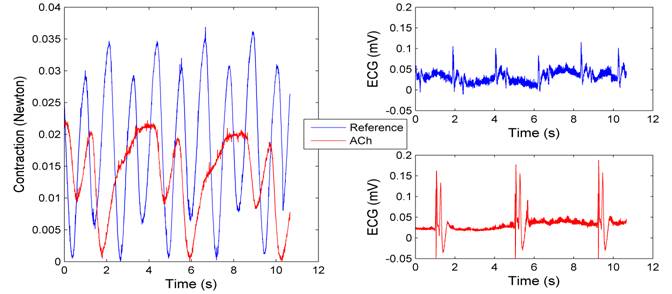
Figure 8: Heart contraction (left) and ECG voltages (right) before and after applying ACh.
3.2.6. Atropine
The heart activity was largely haltered following the application of atropine, as demonstrated by Figure 9. Periodic contractions was replaced by a single, relatively long-standing heart beat during the observing period , implying that the heart beat was greatly suppressed. Correspondingly, the ECG signals indicated that the heart rate fell from 30 BPM to 6-8 BPM.
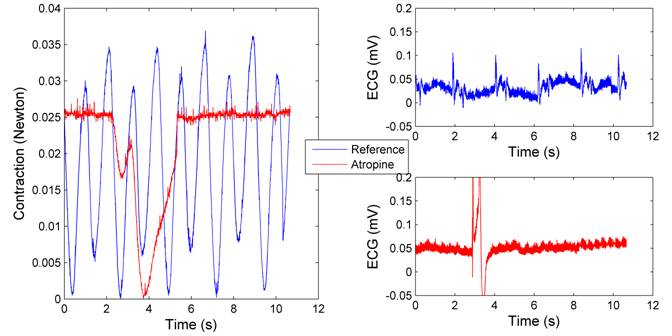
Figure 9:Heart Contraction (left) and ECG voltages (right) before and after applying 1 mg/ml atropine
3.2.7. KCl
The KCl was supposed to kill the heart, terminating all cardiac activities, and the results confirmed this conjecture (Figure 10). Heart contractions nearly disappeared, nor could normal ECG impulses be detected any more.
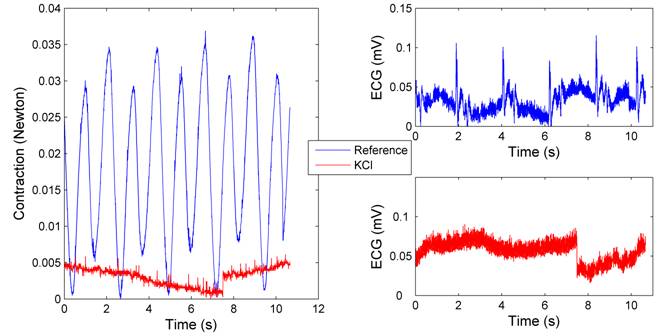
Figure 10: Heart contractions and ECG voltages before and after applying the KCl solution.
4. Discussion
4.1 Response to Stretch Test
Contraction strengths of myocardial muscles are known to increase in proportion with the preload tension or the stroke volume, as described by the Frank-Starling mechanism. Our result (Figure 3) is in accordance with this well-known feature of the heart.
On the other hand, the Frank-Starling mechanism also states that the contraction strength would drop down after the pre-tension reaches an optimal level which gives rise to the maximum contraction strength. We did not observe this reversing phase. The most likely reason is that the strain we exerted had never reached that maximum contraction point.
4.2 Response to Thermal and Chemical Stimulants
Cold Ringerí»s Solution. The Ringerí»s solution is basically used for fluid resuscitation because it is easily absorbed by heart tissues. The use of cold solution tests the thermal response of the heart. Figure 4 shows the contraction force, the heartbeat rate, and the amplitude of ECG voltages all decrease as the temperature drops. The low temperature lowers the concentration of calcium ions in cell fluids, and hence reduces the contraction force. The slowed down heartbeat rate and attenuated ECG signals may be attributed to the slow-down metabolic activities of the heart. Frogs are ectothermic animals which need to maintain low metabolic rates in low temperature.
Cadmium chloride. Cadmium chloride reduces the cardiac output and alters the heartsí» electric activities. Some literatures on internet reported that the cadmium choloride decreases the membrane action potential, which is manifested by a shortened QT interval in the external electrocardiogram. Our empirical findings were in agreement with this statement.
Cadmium is a heavy metal, which in general may inhibit enzymes by reversibly combining with their sulfhydril groups. In this way, Cadmium may block the propagation of activation impulse from the membrane to the contractile elements, thus reducing the heart output.
Epinphrine. Epinphrine is a type of neurotransmitter that boosts the bodyí»s response to external emergency situations. Achieving this function necessarily demands increasing the heart output, and epinephrine increases the stroke volume and heartbeat rates. Figure 7 shows the contraction-strengthening effect by enlarging the stroke volume, but the heart rate was not distinctively promoted in our experiment.
Acyetlcholine (ACh). The ACh is a neurotransmitter that induces contraction of skeletal muscles but decreases contraction of cardiac muscles. The distinction is due to the different receptor structure in their respective muscle fibers. The suppression of contraction was verified in our experiment.
The ACh takes effects by activating potassium channels, especially in the supraventricular parts of the heart. Since the potassium ion has higher concentration in the intracellular space, activating its channels leads to more negative membrane potentials, which is further from the action potential threshold. This process slows down the spontaneous diastolic depolarization in the SA node cells, which in turn decrease the heart rate. The cells in the AV node have similar response to tha of SA node cells, displaying longer refractory period. This mechanism is important for limiting ventricular rate in patients with supraventricular tachyarrhythmias.
Atropine. Atropine is known to increase firing of the SA node and conduction throught the AV node, thus accelerates the heart rate. However, our experiment indicated that the heart rate actually dropped following the application of atropine. One probable explanation is that the heart had been in erratic condition after multiple rounds of mechanical and chemical stimulations. Its abnormal activities were reflected by the extreme noisy baseline ECG signals, as well as the much weaker contractions than at the beginning of our experiments.
KCl. Given the fundamental role of potassium ions in the electrochemical equilibrium of cardiac cells, large dose of potassium ions disrupts inhibits firing of action potentials and thereby arrest the electrical excitability of cardiac cells. As a result, heart beats are quickly terminated and cells die from lack of oxygen and nutrition.