SCI Publications
2013
S.C. Sibole, S.A. Maas, J.P. Halloran, J.A. Weiss, A. Erdemir.
“Evaluation of a post-processing approach for multiscale analysis of biphasic mechanics of chondrocytes,” In Computer Methods in Biomechanical and Biomedical Engineering, Vol. 16, No. 10, pp. 1112--1126. 2013.
DOI: 10.1080/10255842.2013.809711
PubMed ID: 23809004
Understanding the mechanical behaviour of chondrocytes as a result of cartilage tissue mechanics has significant implications for both evaluation of mechanobiological function and to elaborate on damage mechanisms. A common procedure for prediction of chondrocyte mechanics (and of cell mechanics in general) relies on a computational post-processing approach where tissue-level deformations drive cell-level models. Potential loss of information in this numerical coupling approach may cause erroneous cellular-scale results, particularly during multiphysics analysis of cartilage. The goal of this study was to evaluate the capacity of first- and second-order data passing to predict chondrocyte mechanics by analysing cartilage deformations obtained for varying complexity of loading scenarios. A tissue-scale model with a sub-region incorporating representation of chondron size and distribution served as control. The post-processing approach first required solution of a homogeneous tissue-level model, results of which were used to drive a separate cell-level model (same characteristics as the sub-region of control model). The first-order data passing appeared to be adequate for simplified loading of the cartilage and for a subset of cell deformation metrics, for example, change in aspect ratio. The second-order data passing scheme was more accurate, particularly when asymmetric permeability of the tissue boundaries was considered. Yet, the method exhibited limitations for predictions of instantaneous metrics related to the fluid phase, for example, mass exchange rate. Nonetheless, employing higher order data exchange schemes may be necessary to understand the biphasic mechanics of cells under lifelike tissue loading states for the whole time history of the simulation.
2012
G.A. Ateshian, S.A. Maas, J.A. Weiss.
“Solute transport across a contact interface in deformable porous media,” In Journal of Biomechanics, Vol. 45, No. 6, pp. 1023-–1027. 2012.
DOI: 10.1016/j.jbiomech.2012.01.003
A finite element formulation of neutral solute transport across a contact interface between deformable porous media is implemented and validated against analytical solutions. By reducing the integral statements of external virtual work on the two contacting surfaces into a single contact integral, the algorithm automatically enforces continuity of solute molar flux across the contact interface, whereas continuity of the effective solute concentration (a measure of the solute mechano-chemical potential) is achieved using a penalty method. This novel formulation facilitates the analysis of problems in biomechanics where the transport of metabolites across contact interfaces of deformable tissues may be of interest. This contact algorithm is the first to address solute transport across deformable interfaces, and is made available in the public domain, open-source finite element code FEBio (http://www.febio.org).
Keywords: FEBio, Finite element modeling, Contact mechanics, Solute transport, Porous media, Biphasic theory
C.C. Chang, L. Krishnan, S.S. Nunes, K.H. Church, L.T. Edgar, E.D. Boland, J.A. Weiss, S.K. Williams, J.B. Hoying.
“Determinants of microvascular network topology in implanted neovasculatures,” In Arteriosclerosis, Thrombosis, and Vascular Biology, Vol. 32, No. 1, pp. 5--14. 2012.
DOI: 10.1161/ATVBAHA.111.238725
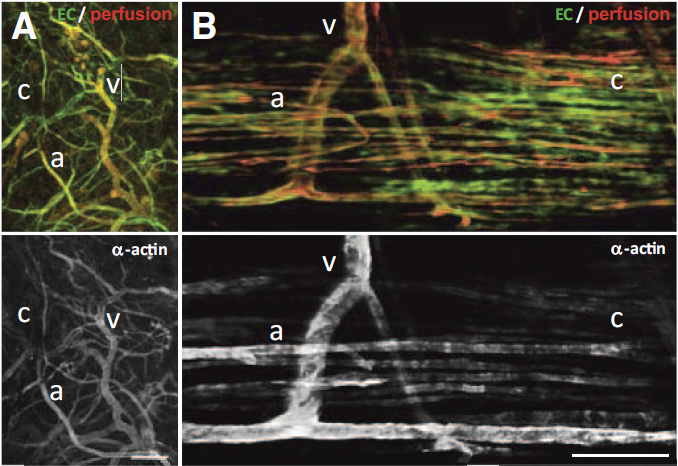
Objective
During neovascularization, the end result is a new functional microcirculation composed of a network of mature microvessels with specific topologies. Although much is known concerning the mechanisms underlying the initiation of angiogenesis, it remains unclear how the final architecture of microcirculatory beds is regulated. To begin to address this, we determined the impact of angiogenic neovessel prepatterning on the final microvascular network topology using a model of implant neovascularization.
Methods and Results
We used 3D direct-write bioprinting or physical constraints in a manner permitting postangiogenesis vascular remodeling and adaptation to pattern angiogenic microvascular precursors (neovessels formed from isolated microvessel segments) in 3D collagen gels before implantation and subsequent network formation. Neovasculatures prepatterned into parallel arrays formed functional networks after 4 weeks postimplantation but lost the prepatterned architecture. However, maintenance of uniaxial physical constraints during postangiogenesis remodeling of the implanted neovasculatures produced networks with aligned microvessels, as well as an altered proportional distribution of arterioles, capillaries, and venules.
Conclusion
Here we show that network topology resulting from implanted microvessel precursors is independent from prepatterning of precursors but can be influenced by a patterning stimulus involving tissue deformation during postangiogenesis remodeling and maturation.
J.P. Halloran, S. Sibole, C.C. Van Donkelaar, M.C. Van Turnhout, O.W. Oomens, J.A. Weiss, F. Guilak, A. Erdemir.
“Multiscale mechanics of articular cartilage: potentials and challenges of coupling musculoskeletal, joint, and microscale computational models,” In Annals of Biomedical Engineering, Vol. 40, No. 11, pp. 2456--2474. 2012.
PubMed ID: 10.1007/s10439-012-0598-0
Articular cartilage experiences significant mechanical loads during daily activities. Healthy cartilage provides the capacity for load bearing and regulates the mechanobiological processes for tissue development, maintenance, and repair. Experimental studies at multiple scales have provided a fundamental understanding of macroscopic mechanical function, evaluation of the micromechanical environment of chondrocytes, and the foundations for mechanobiological response. In addition, computational models of cartilage have offered a concise description of experimental data at many spatial levels under healthy and diseased conditions, and have served to generate hypotheses for the mechanical and biological function. Further, modeling and simulation provides a platform for predictive risk assessment, management of dysfunction, as well as a means to relate multiple spatial scales. Simulation-based investigation of cartilage comes with many challenges including both the computational burden and often insufficient availability of data for model development and validation. This review outlines recent modeling and simulation approaches to understand cartilage function from a mechanical systems perspective, and illustrates pathways to associate mechanics with biological function. Computational representations at single scales are provided from the body down to the microstructure, along with attempts to explore multiscale mechanisms of load sharing that dictate the mechanical environment of the cartilage and chondrocytes.
B.J. Hansen, M.D. Harris, L.A. Anderson, C.L. Peters, J.A. Weiss, A.E. Anderson.
“Correlation between radiographic measures of acetabular morphology with 3D femoral head coverage in patients with acetabular retroversion,” In Acta Orthopaedica, Vol. 83, No. 3, pp. 233--239. 2012.
DOI: 10.3109/17453674.2012.684138
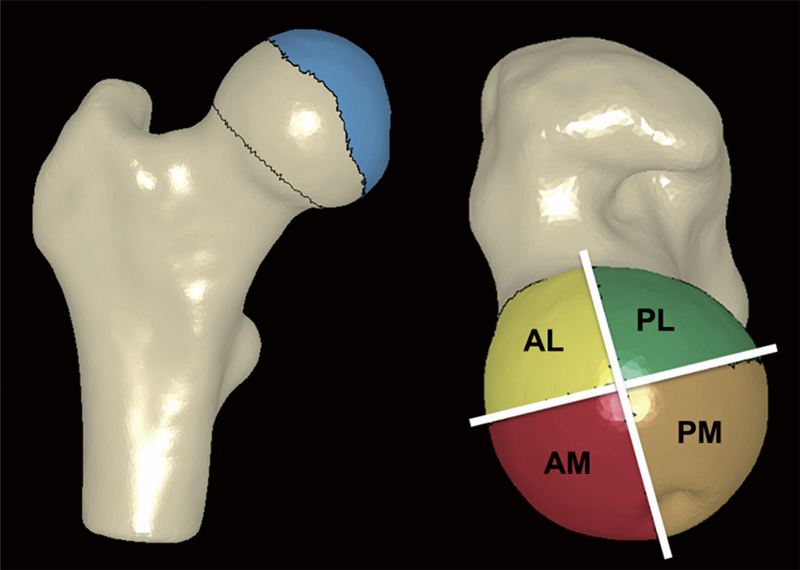
Background and purpose
Acetabular retroversion may result in anterior acetabular over-coverage and posterior deficiency. It is unclear how standard radiographic measures of retroversion relate to measurements from 3D models, generated from volumetric CT data. We sought to: (1) compare 2D radiographic measurements between patients with acetabular retroversion and normal control subjects, (2) compare 3D measurements of total and regional femoral head coverage between patients and controls, and (3) quantify relationships between radiographic measurements of acetabular retroversion to total and regional coverage of the femoral head.
Patients and methods
For 16 patients and 18 controls we measured the extrusion index, crossover ratio, acetabular angle, acetabular index, lateral center edge angle, and a new measurement termed the "posterior wall distance". 3D femoral coverage was determined from volumetric CT data using objectively defined acetabular rim projections, head-neck junctions, and 4 anatomic regions. For radiographic measurements, intra-observer and inter-observer reliabilities were evaluated and associations between 2D radiographic and 3D model-based measures were determined.
Results
Compared to control subjects, patients with acetabular retroversion had a negative posterior wall distance, increased extrusion index, and smaller lateral center edge angle. Differences in the acetabular index between groups approached statistical significance. The acetabular angle was similar between groups. Acetabular retroversion was associated with a slight but statistically significant increase in anterior acetabular coverage, especially in the anterolateral region. Retroverted hips had substantially less posterior coverage, especially in the posterolateral region.
Interpretation
We found that a number of 2D radiographic measures of acetabular morphology were correlated with 3D model-based measures of total and regional femoral head coverage. These correlations may be used to assist in the diagnosis of retroversion and for preoperative planning.
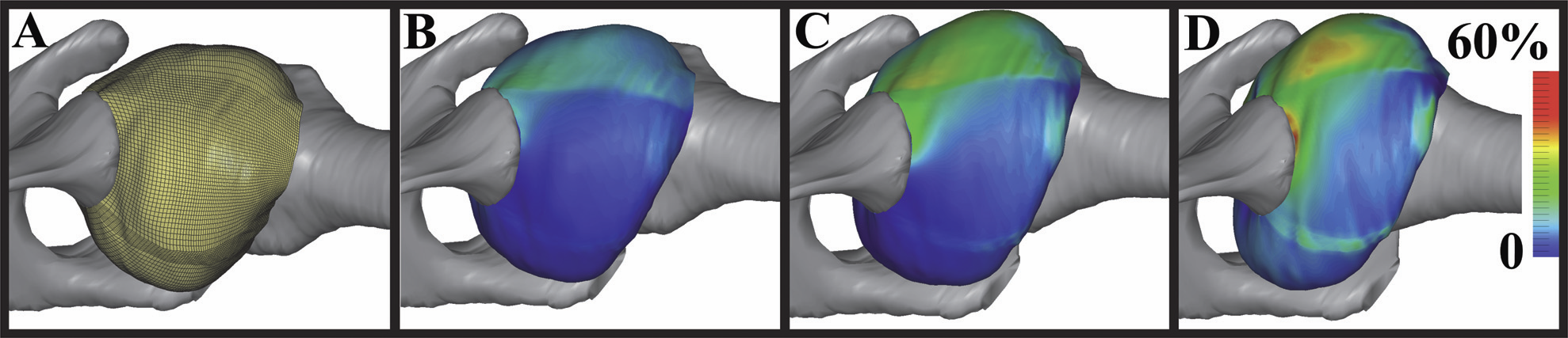
M.D. Harris, A.E. Anderson, C.R. Henak, B.J. Ellis, C.L. Peters, J.A. Weiss.
“Finite element prediction of cartilage contact stresses in normal human hips,” In Journal of Orthopaedic Research, Vol. 30, No. 7, pp. 1133--1139. 2012.
DOI: 10.1002/jor.22040

Keywords: hip, finite element, biomechanics, cartilage contact stresses, cartilage pressure
S.A. Maas, B.J. Ellis, G.A. Ateshian, J.A. Weiss.
“FEBio: Finite elements for biomechanics,” In Journal of Biomechanical Engineering, Vol. 134, No. 1, pp. 011005. 2012.
DOI: 10.1115/1.4005694
PubMed ID: 22482660
2011
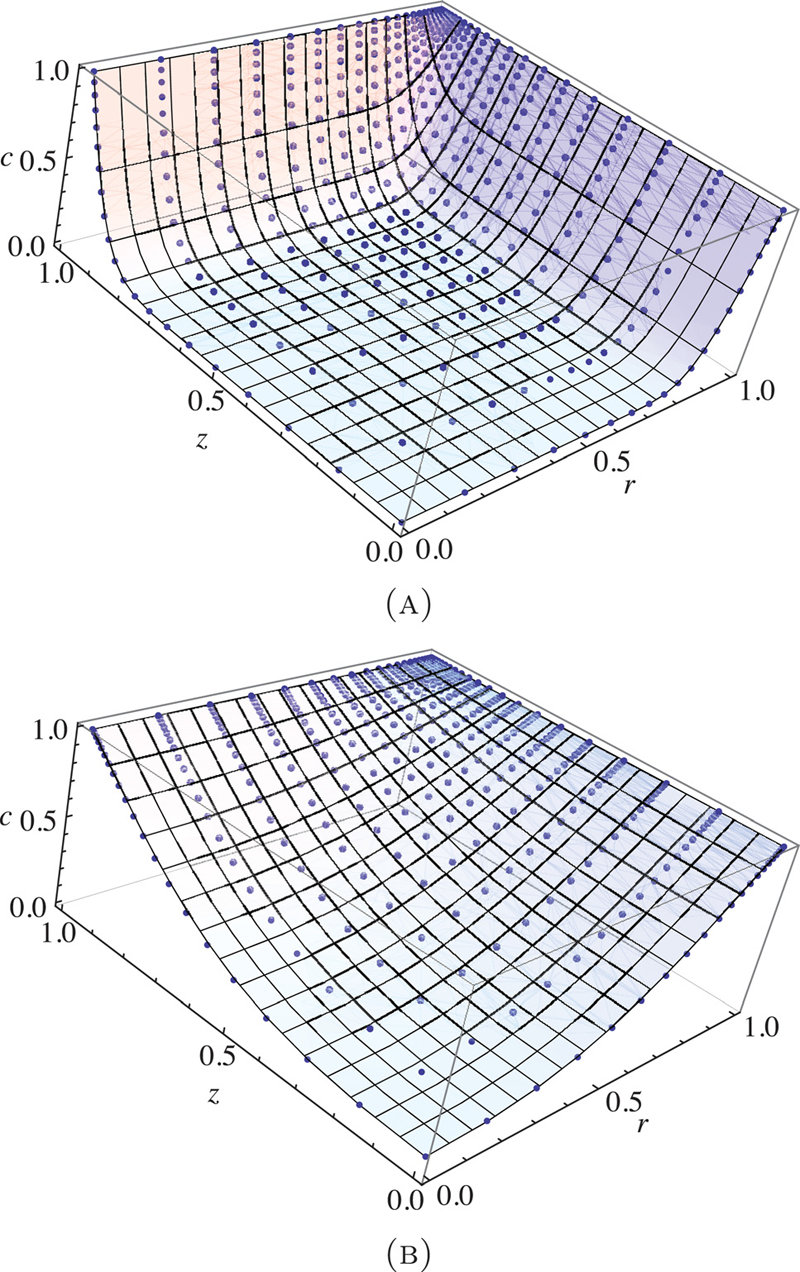
G.A. Ateshian, M.B. Albro, S.A. Maas, J.A. Weiss.
“Finite element implementation of mechanochemical phenomena in neutral deformable porous media under finite deformation,” In Journal of Biomechanical Engineering, Vol. 133, No. 8, 2011.
DOI: 10.1115/1.4004810
N.J. Drury, B.J. Ellis, J.A. Weiss, P.J. McMahon, R.E. Debski.
“Finding consistent strain distributions in the glenohumeral capsule between two subjects: Implications for development of physical examinations,” In Journal of Biomechanics, Vol. 44, No. 4, pp. 607-613. February, 2011.
DOI: 10.1016/j.jbiomech.2010.11.018
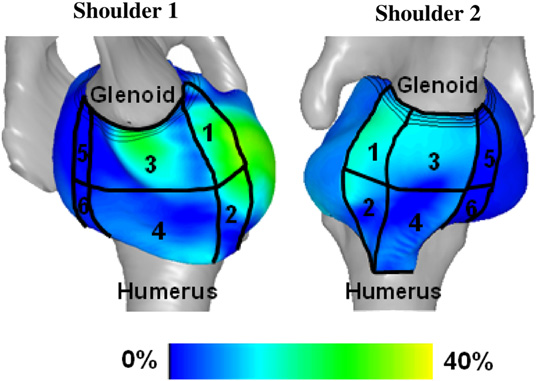
J.M. Elkins, J.S. Stroud, M.J. Rudert, Y. Tochigi, D.R. Pedersen, B.J. Ellis, J.J. Callaghan, J.A. Weiss, T.D. Brown.
“The capsule's contribution to total hip construct stability - a finite element analysis,” In Journal of Orthopedic Research, Vol. 29, No. 11, Note: William Harris, MD Award, pp. 1642--1648. November, 2011.
DOI: 10.1002/jor.21435
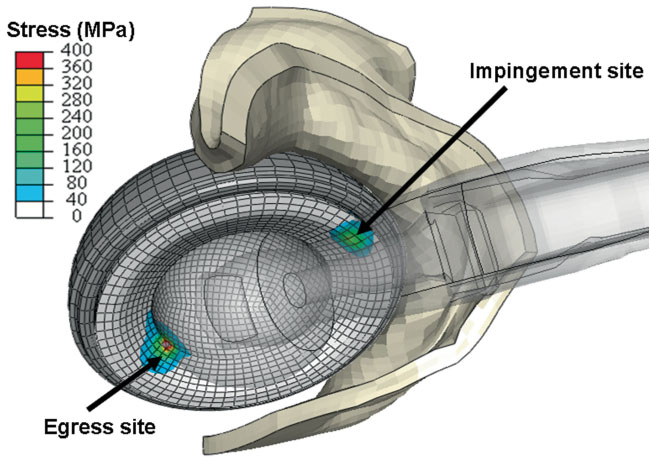
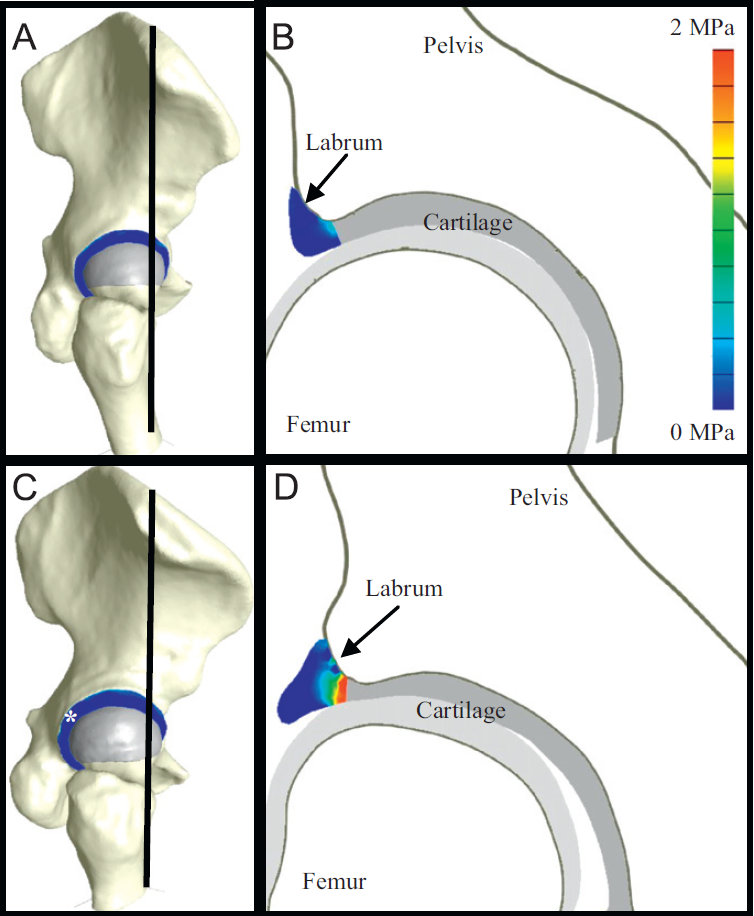
C.R. Henak, B.J. Ellis, M.D. Harris, A.E. Anderson, C.L. Peters, J.A. Weiss.
“Role of the acetabular labrum in load support across the hip joint,” In Journal of Biomechanics, Vol. 44, No. 12, pp. 2201-2206. 2011.
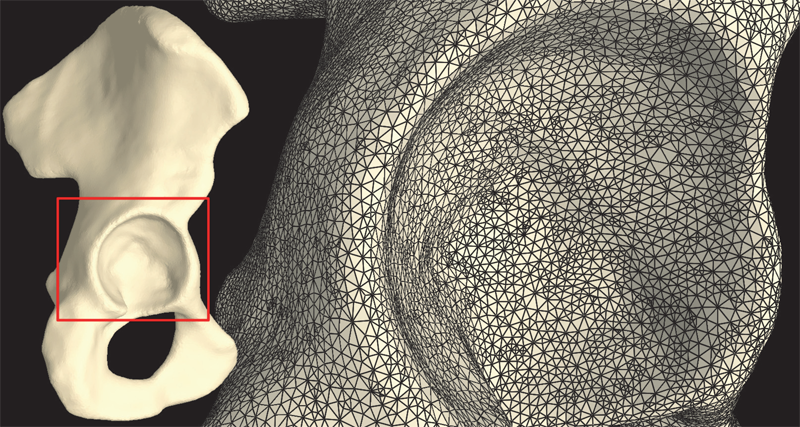
S.A. Maas, B.J. Ellis, D.S. Rawlins, L.T. Edgar, C.R. Henak, J.A. Weiss.
“Implementation and Verification of a Nodally-Integrated Tetrahedral Element in FEBio,” SCI Technical Report, No. UUSCI-2011-007, SCI Institute, University of Utah, 2011.
Keywords: MRL
2010
A.E. Anderson, B.J. Ellis, S.A. Maas, J.A. Weiss.
“Effects of idealized joint geometry on finite element predictions of cartilage contact stresses in the hip,” In Journal of Biomechanics, Vol. 43, No. 7, pp. 1351--1357. May, 2010.
Computational models may have the ability to quantify the relationship between hip morphology, cartilage mechanics and osteoarthritis. Most models have assumed the hip joint to be a perfect ball and socket joint and have neglected deformation at the bone-cartilage interface. The objective of this study was to analyze finite element (FE) models of hip cartilage mechanics with varying degrees of simplified geometry and a model with a rigid bone material assumption to elucidate the effects on predictions of cartilage stress. A previously validated subject-specific FE model of a cadaveric hip joint was used as the basis for the models. Geometry for the bone-cartilage interface was either: (1) subject-specific (i.e. irregular), (2) spherical, or (3) a rotational conchoid. Cartilage was assigned either a varying (irregular) or constant thickness (smoothed). Loading conditions simulated walking, stair-climbing and descending stairs. FE predictions of contact stress for the simplified models were compared with predictions from the subject-specific model. Both spheres and conchoids provided a good approximation of native hip joint geometry (average fitting error ∼0.5 mm). However, models with spherical/conchoid bone geometry and smoothed articulating cartilage surfaces grossly underestimated peak and average contact pressures (50% and 25% lower, respectively) and overestimated contact area when compared to the subject-specific FE model. Models incorporating subject-specific bone geometry with smoothed articulating cartilage also underestimated pressures and predicted evenly distributed patterns of contact. The model with rigid bones predicted much higher pressures than the subject-specific model with deformable bones. The results demonstrate that simplifications to the geometry of the bone-cartilage interface, cartilage surface and bone material properties can have a dramatic effect on the predicted magnitude and distribution of cartilage contact pressures in the hip joint.
Keywords: mrl
G.A. Ateshian, S.A. Maas, J.A. Weiss.
“Finite element algorithm for frictionless contact of porous permeable media under finite deformation and sliding,” In Journal of Biomechanical Engineering, Vol. 132, No. 6, Note: Cover article, 2010.
N.J. Drury, B.J. Ellis, J.A. Weiss, P.J. McMahon, R.E. Debski.
“The impact of glenoid labrum thickness and modulus on labrum and glenohumeral capsule function,” In Journal of Biomechanical Engineering, Vol. 132, No. 12, Note: Awarded 2010 Skalak Best Paper!, pp. 121003--121010. 2010.
DOI: 10.1115/1.4002622
H.B. Henninger, C.J. Underwood, G.A. Ateshian, J.A. Weiss.
“Effect of sulfated glycosaminoglycan digestion on the transverse permeability of medial collateral ligament.,” In Journal of Biomechanics, Vol. 43, pp. 2567--2573. 2010.
S.P. Reese, S.A. Maas, J.A. Weiss.
“Micromechanical models of helical superstructures in ligament and tendon fibers predict large poisson's ratios,” In Journal of Biomechanics, Vol. 43, No. 7, pp. 1394--1400. 2010.
2009
H.B. Henninger, S.A. Maas, J.H. Shepherd, S. Joshi, J.A. Weiss.
“Transversely Isotropic Distribution of Sulfated Glycosaminoglycans in Human Medial Collateral Ligament: A Quantitative Analysis,” In Journal of Structural Biology, Vol. 165, pp. 176-183. 2009.
PubMed ID: 19126431
H.B. Henninger, S.P. Reese, A.E. Anderson, J.A. Weiss.
“Validation of computational models in biomechanics,” In Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine, Vol. 224, No. 7, SAGE Publications, pp. 801--812. 2009.
S.A. Maas, B.J. Ellis, D.S. Rawlins, J.A. Weiss.
“A Comparison of FEBio, ABAQUS, and NIKE3D Results for a Suite of Verification Problems,” SCI Technical Report, No. UUSCI-2009-009, SCI Institute, University of Utah, 2009.
Page 2 of 6
