 |
| Transcranial Magnetic Stimulation (TMS) of the human motor cortex. |
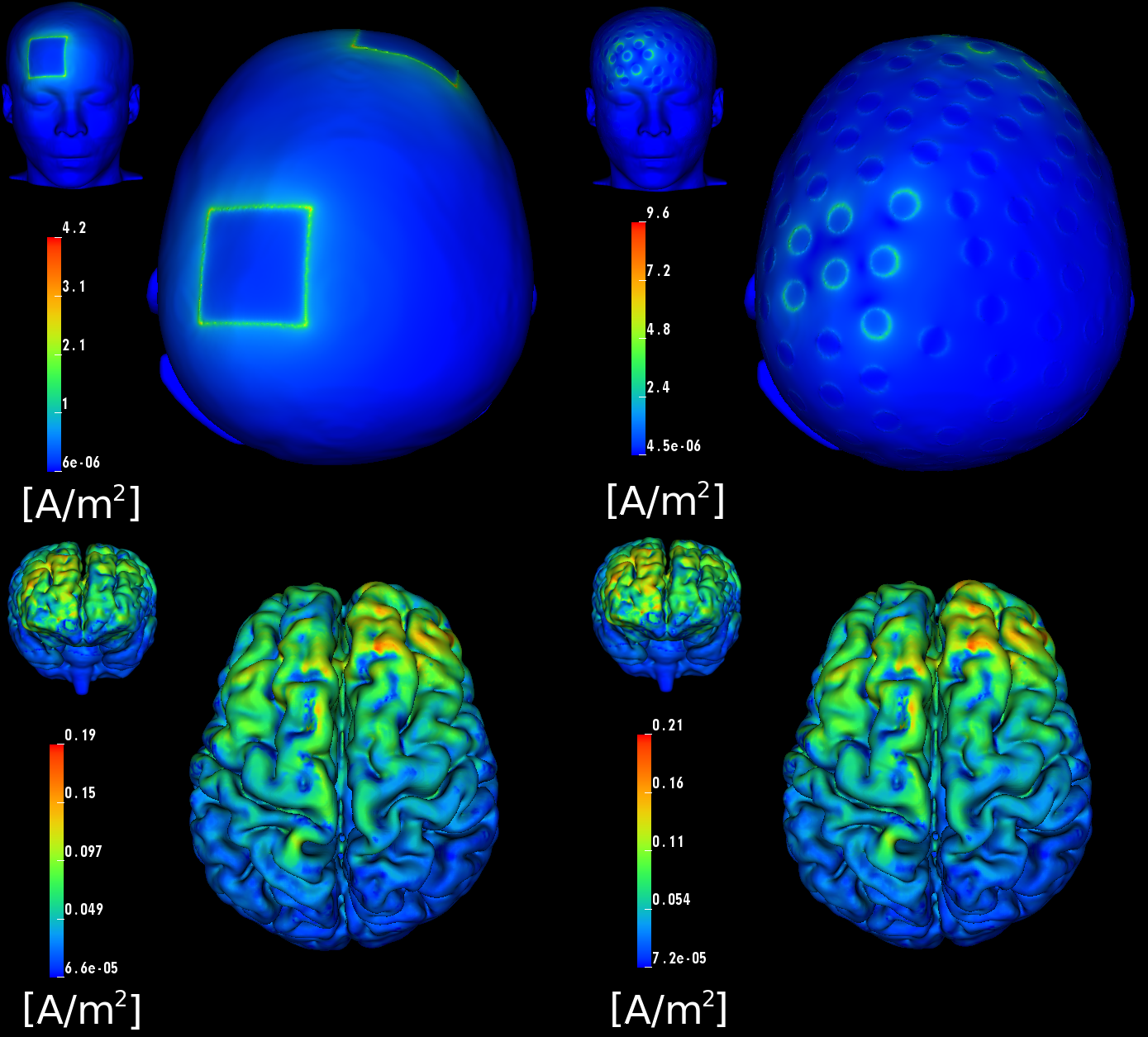
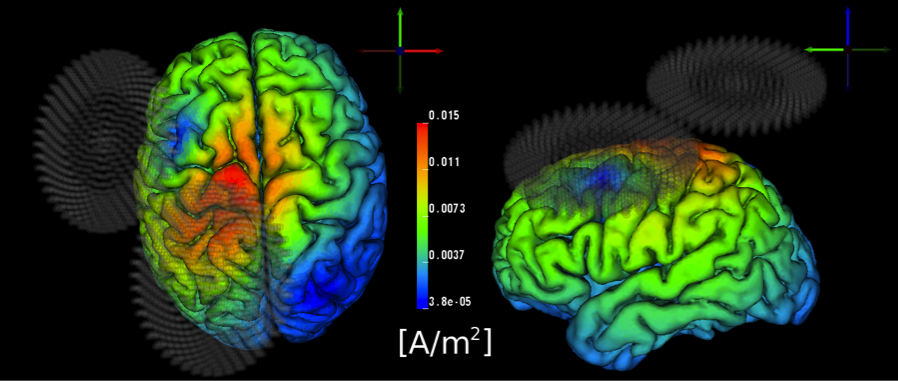
In close collaboration with our DBP partner Don Tucker and his co-workers at EGI, Inc. and the University of Oregon, including Pavel Govyadinov, Sergei Turovets, Phan Luu, Adnan Salman, Daniel Ellsworth, and Allen Malony, we have developed tools at CIBC to model and optimize such brain stimulation. Although we have developed modeling software for the stimulation by means of magnetic fields, TMS (see Figure 4.6), we have concentrated mainly on modeling electrical stimulation, tDCS. Specifically, we have studied the potential advantages of using a high-density array of 128–256 electrodes originally designed to record electroencephalography (EEG) signals, for tDCS stimulation. Such a configuration contrasts with the conventional commercial systems that use only two large "patch" electrodes. Our postdoctoral fellow at CIBC, Moritz Dannhauer, has led our efforts on this project. He has created two highly accurate geometric head models based on anatomical and functional images provided by EGI, including anatomical MR, diffusion MR, and CT. These models include anisotropic conductivity specified through measurement with a technique known as bounded electrical impedance tomography (bEIT), which uses controlled current injection and simultaneous voltage measurements to estimate tissue conductivities. The improved precision of this model over any previously published was further aided by inclusion not only of diffusion imaging data (a special form of MRI that reveals anisotropic conductivity), but also of bone density (X-ray attenuation) estimates of skull conductivity in the EGI head model (protected by US Patent 6,529,759).
Constructing the head models involved the use of both automated and manual segmentation methods, both guided and integrated using CIBC's Seg3D software. After segmentation followed tetrahedral meshing (described in more detail in the first highlight of this section) of the brain which carefully respected internal organ boundaries, and which we carried out using CIBC's BioMesh3D and Cleaver meshing tools. Once the model was constructed, we applied our Finite Element Modeling software in SCIRun to simulate the effects of stimulation with various electrode configurations and current injection patterns.
Dr. Dannhauer has carried out a large number of stimulations in the context of the EGI collaboration, including comparison of stimulation with a high-density array to that achieved with standard patch electrodes (see Figure 4.7). One particularly interesting result of our simulations is that the current, rather than being distributed over the surface of the large patch electrodes, is exclusively focused on their perimeter, meaning that peak current density (which can lead directly to subject discomfort) is higher, rather than lower, with the conventional patch electrodes compared to that with the high density electrode array. This perimeter current concentration is actually predicted by well known physical principles, but has not been recognized in the tDCS community.
Once the FEM simulations were in place, our team, primarily Seyhmus Guler, a CIBC PhD student at Northeastern University, developed an extremely efficient and highly relevant optimization approach that starts with the specification of a region of interest (ROI) that we desire to target with stimulation, along with a desired current field direction distributed within the ROI. It has been widely reported that the direction of the current through a particular brain region has great impact on the stimulus effect, even to the point that reversing current direction can reverse the effect from stimulation to inhibition or vice versa. In addition, the user can specify a number of safety constraints, including both total and electrode-specific current limits and a limit on the total current magnitude in the rest of the brain. The optimization then determines which electrodes to use, and how much current (both amplitude and direction) to apply on each of those electrodes, so as to maximize current flow in the specified directions in the ROI while respecting the safety constraints. We have achieved results with a surprisingly degree of localization of the current in the brain, especially in superficial regions of the cortex but even, to some extent, in deeper regions. In particular, we found that, in contrast to what is possible with conventional electrodes, carefully patterned tDCS with multiple electrodes from the dense array can target focal sites both at the outer (gyral) surface of the cortex as well as deeper sites, such as the anterior cingulate cortex.
More recently, we have begun to collaborate with a number of other tDCS and TMS investigators in groups in the US and Europe, including Drs. A. Pascual-Leone, M. Halko, and B. Manor, at Harvard Medical School's Beth Israel Deaconess Hospital in Boston, Prof. Dagmar Sternad at Northeastern University, and several groups in Germany. This collaboration has already resulted in a paper recently accepted for publication in NeuroImage and several other manuscripts are currently in preparation or under review. As we move forward, we will concentrate on validation and extensions of these approaches, some of which are already underway. These extensions include comparisons of our simulations to experimental results, approximation of both the optimal solutions to suboptimal approximations that do not require independent control of each electrode in the high-density array, as well as optimizing the placement of more conventional electrodes.